
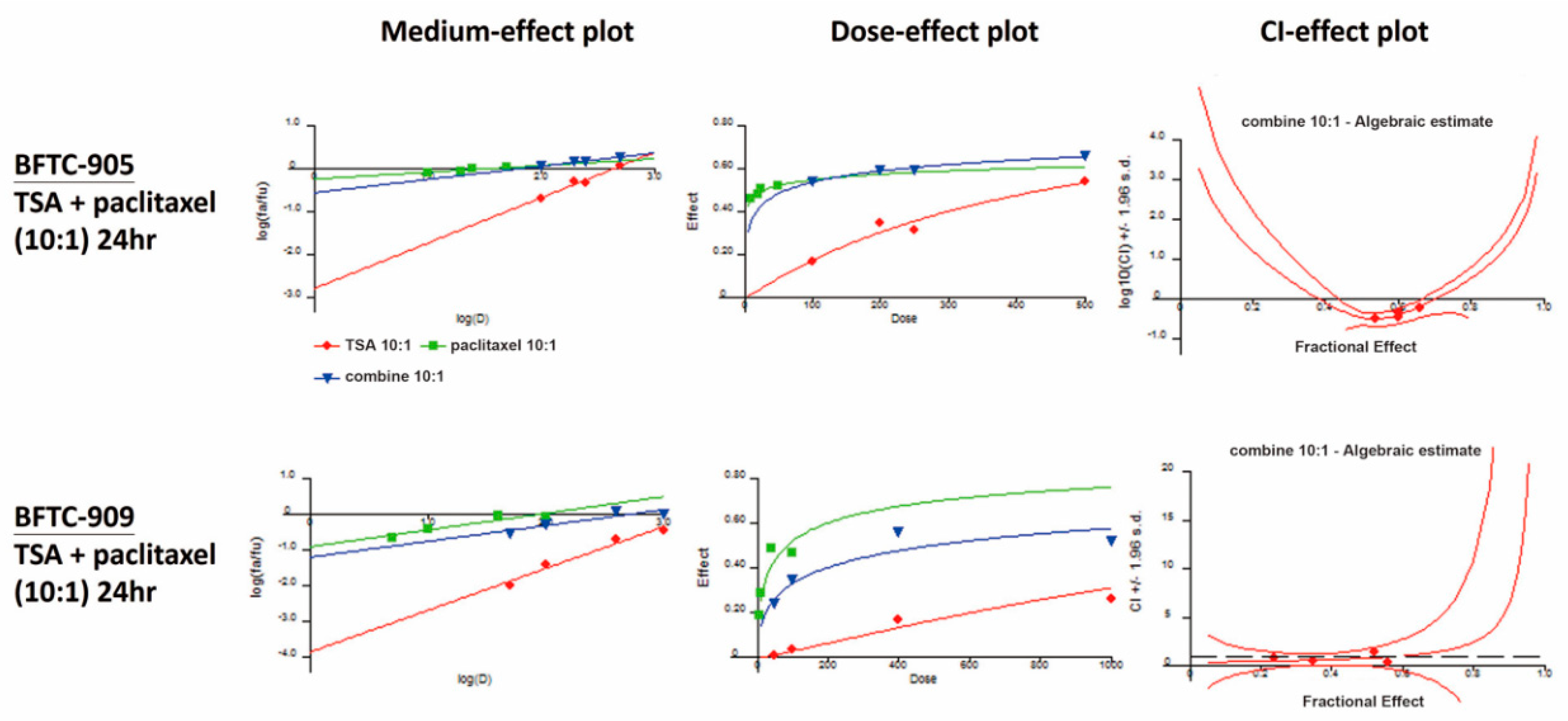
Results: Fusion peptides T-20 (class 1) and (CCIZN17) 3 (class 2) work synergistically in inhibiting the entry of HIV-1 89.6 with the CI values reaching 0.37 and 0.32 at IC 50 and IC 90, respectively, and a similar degree of neutralization synergy was demonstrated between the MAb D5 that targets the hydrophobic pocket region of the NHR, and 2F5, a well-characterized MAb which targets the C-terminal end of CHR and the MPER, with the CIs being 0.39 and 0.32 at IC 50 and IC 90, respectively. A combination index (CI) is calculated to serve as an indicator of synergy, with values of less than 1, equal to 1, and greater than 1 indicating synergy, additive effect, and antagonism, respectively. The antiviral or neutralization synergy was assessed by using the computer program CalcuSyn. P4/R5 cells were infected with the HIV-1 89.6 mixed with titrations of peptides and/or MAbs either alone or in combination, and β-galactosidase activity indicative of viral replication was determined. This study is directed toward examining potential synergistic effects between class 1 and class 2 entry inhibitors against primary HIV-1 isolates. Based on the observations of this study, we suggest that combined administration of these two drugs might be considered a novel therapeutic regimen for treating MDS.Background: Peptide-based fusion inhibitors and monoclonal antibodies (MAbs) can block HIV-1 entry by binding to either the NHR (class 1 inhibitors) or the CHR/MPER region (class 2 inhibitors) of the gp41 and preventing its intramolecular folding that is necessary for viral entry. Additionally, combination of 2.5 μmol/L DAC and 5 μmol/L ATO led to a significantly higher apoptosis rate and more significantly decreased the Bcl2/Bax ratio than either compound alone ( P < 0.001). The results showed that DAC and/or ATO can inhibit the proliferation of SKM-1 cells and demonstrated significant synergy between the two agents (CI < 1). Furthermore, we determined apoptosis and measured the mRNA expression level of two genes that are considered main regulators of the apoptosis process. To study the combined effects and mechanism of decitabine (DAC) and arsenic trioxide (ATO) on the human myelodysplastic cell line SKM-1,we used the MTS assay and CalcuSyn software to determine the cytotoxicity and potential synergistic effects, respectively. Despite recent advances in the treatment of myelodysplastic syndrome (MDS), single-agent clinical effects remain unsatisfactory, and decitabine monotherapy is also associated with a relatively low rate of complete remission.


 0 kommentar(er)
0 kommentar(er)
